Isalathiso (usetyenziso oluvunyiweyo): Ngo-2019, i-FDA yaluvuma unyango olufunyenweyo, oluqhelekileyo lwe-hypoactive sex disorder disorder (HSDD) kubasetyhini abangaphambi kokuya esikhathini xa imeko ibangela uxinzelelo oluphawulweyo kwaye ingekho ngenxa yezinye iimeko zonyango/zengqondo okanye iziphumo ebezingalindelekanga zeziyobisi.
Indlela yokwenza
I-PT-141 yi-agonist ye-melanocortin receptor (ikakhulukazi i-MC4 receptor) ehlengahlengisa umnqweno wesini ngokusebenzisa iindlela zenkqubo ye-nervous central.
Ngokungafani ne-PDE5 inhibitors (umzekelo, i-sildenafil), echaphazela kakhulu imithwalo yegazi, i-PT-141 isebenza ngokuyintloko ukuchaphazela ukukhuthaza ngokwesondo kunye nokuvusa.
Pharmacology & Dosing
Ulawulo: Isitofu esingaphantsi kwesikhumba, njengoko kufuneka (kwimfuno).
Idosi evunyiweyo: 1.75 mg sc
I-Pharmacokinetics:
Tmax ≈ ~60 imizuzu
t½ ≈ iiyure ezi-2–3
Iziphumo zingahlala iiyure ezininzi, kwezinye iingxelo ukuya kuthi ga kwiiyure ezili-16.
Ukusebenza kweKlinikhi (Izilingo zeSigaba se-III - XHUMANA, iiveki ze-24, ii-RCTs)
Iziphelo eziphambili:
Isalathiso soMsebenzi weSexual owasetyhini-Idomeyini yoMnqwenelo (FSFI-D)
Isikali soKubandezeleka ngokweSondo kwabasetyhini (FSDS-DAO)
Iziphumo eziphambili (izifundo ezidityanisiweyo 301 + 302):
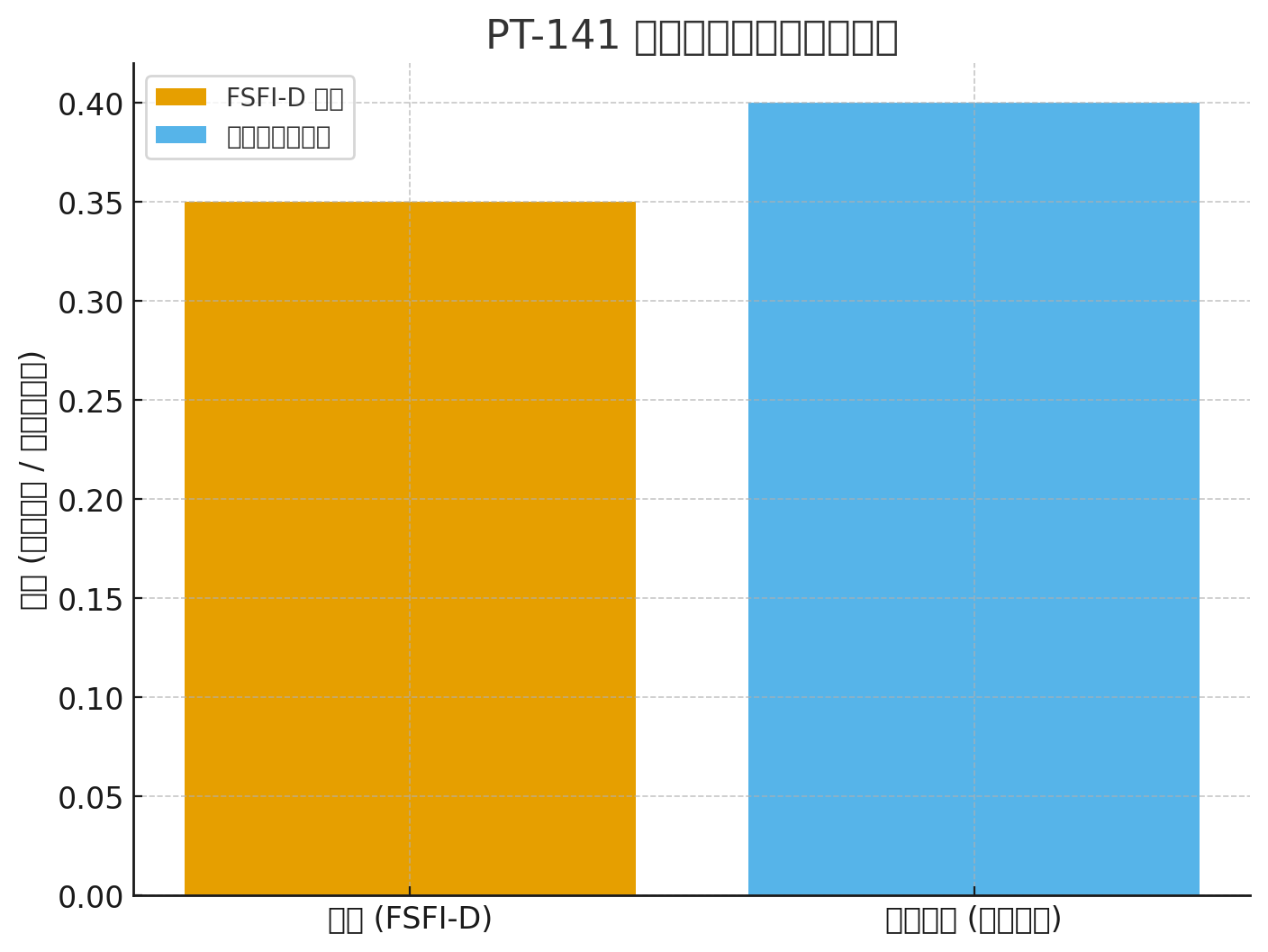
Ukuphuculwa kwe-FSFI-D: + 0.35 vs placebo (P <0.001)
Ukunciphisa amanqaku e-FSDS-DAO: -0.33 vs placebo (P<0.001)
Ezinye iziphelo: Iziphumo ezixhasayo (amanqaku omsebenzi wesondo, ukwaneliseka okuxelwe ngesigulane) zihamba kakuhle, kodwa iziganeko zesondo ezanelisayo (SSEs) azizange zihlale zibonisa ukungafani okuphawulekayo okuhambelanayo.
Iziganeko ezimbi (ezona zixelwa rhoqo kulingo)
Ixhaphakile (≥10%):
Isicaphucaphu (~ 30–40%; ukuya kuthi ga kwi-40% exelwe kulingo)
Ukugungxula (≥10%)
Intloko ebuhlungu (≥10%)
Iziphumo zentliziyo:
Ukunyuka okwethutyana kwixinzelelo lwegazi kunye notshintsho kwisantya sentliziyo kwabonwa, ngokuqhelekileyo kusonjululwe kwiiyure ezimbalwa.
I-Contraindicated okanye isetyenziswe ngokuqaphela kwizigulane ezine-hypertension engalawulwayo okanye isifo senhliziyo.
Isibindi: Iingxelo ezinqabileyo zokuphakama kwe-enzyme yesibindi; Iingxelo zemeko ezinqabileyo kakhulu zibonisa ukwenzakala okubukhali kwesibindi okunokwenzeka, kodwa akuqhelekanga.
Ukhuseleko Lwexesha elide (Uphononongo olongezelelweyo)
Uphononongo olwandisiweyo lweeveki ezingama-52 oluvulelekileyo lufumene uphuculo oluzinzileyo kumnqweno kungekho miqondiso mitsha yokhuseleko.
Iprofayili yokhuseleko lwexesha elide ithathwa njengenyamezeleka ngokubanzi, kunye nemiba ephambili yokunyamezela iseyiziphumo ezibi zexesha elifutshane ezifana nesicaphucaphu.
Amanqaku okusetyenziswa angundoqo
Abemi abavunyiweyo balinganiselwe: Kuphela kubafazi be-premenopausal abane-HSDD efunyenweyo, eqhelekileyo.
Ayivunywanga ngokubanzi kumadoda (i-ED okanye umnqweno ophantsi emadodeni uhlala uphanda).
Ukuhlolwa kokhuseleko kubalulekile: Uxinzelelo lwegazi, isifo senhliziyo, kunye nembali yesibindi kufuneka ihlolwe ngaphambi kokumisela.
IsiShwankathelo seDatha esiKhawulezayo
Imvume ye-FDA: 2019 (Vyleesi).
I-Dose: I-1.75 mg i-injection subcutaneous, ngokufunwa.
PK: Tmax ~ 60 min; t½ 2–3 y; iziphumo ukuya kwi ~ 16 h.
Ukusebenza kakuhle (Isigaba III, sidityanisiwe):
FSFI-D: +0.35 (P <.001)
I-FSDS-DAO: −0.33 (P <.001)
Iziganeko ezimbi:
Isicaphucaphu: ukuya kuthi ga kwi-40%
Ukugungxula: ≥10%
Intloko ebuhlungu: ≥10%
Ukunyuka kweBP okwethutyana kuphawuliwe.
Uthelekiso lweTheyibhile kunye neGrafu (isiShwankathelo)
| Isifundo / Uhlobo lweDatha | Isiphelo / Umlinganiselo | Ixabiso / Inkcazo |
|---|---|---|
| Isigaba III (301+302 sidityanisiwe) | FSFI-D (isizinda somnqweno) | + 0,35 vs placebo (P <0.001); I-FSDS-DAO -0.33 |
| Iziganeko ezimbi | Isicaphucaphu, ukugungxula, intloko ebuhlungu | I-nausea ~ 30-40% (ubuninzi ~ 40%); ukugungxula ≥10%; intloko ebuhlungu ≥10% |
Ixesha lokuposa: Sep-30-2025